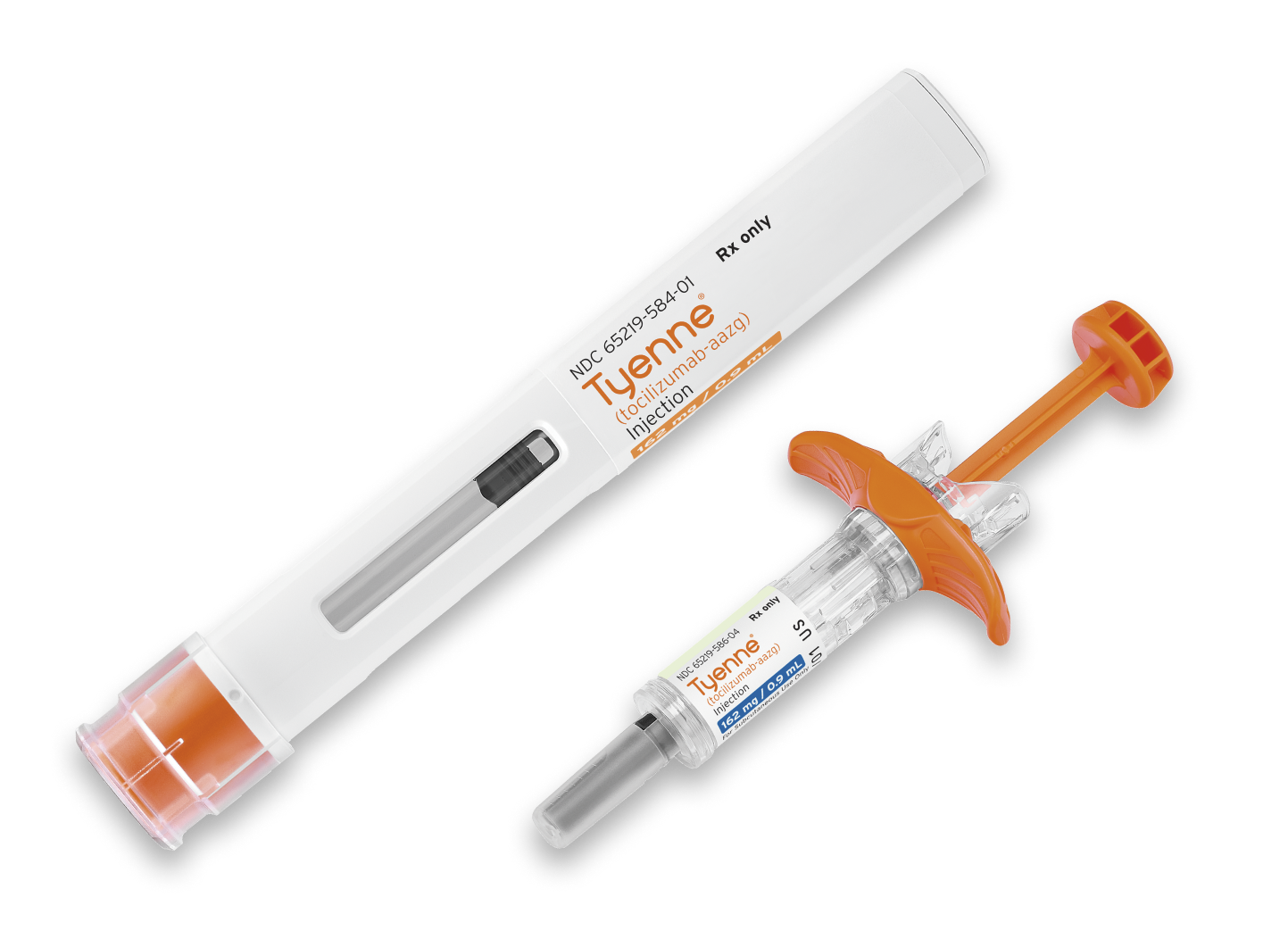
INDICATIONS
-
TYENNE (tocilizumab-aazg) is indicated for the treatment of adult patients with moderately to severely active rheumatoid arthritis (RA) who have had an inadequate response to one or more Disease-Modifying Anti-Rheumatic Drugs (DMARDs).
-
TYENNE (tocilizumab-aazg) is indicated for the treatment of giant cell arteritis (GCA) in adult patients.
-
TYENNE (tocilizumab-aazg) is indicated for the treatment of active polyarticular juvenile idiopathic arthritis (PJIA) in patients 2 years of age and older.
-
TYENNE (tocilizumab-aazg) is indicated for the treatment of active systemic juvenile idiopathic arthritis (SJIA) in patients 2 years of age and older.
IMPORTANT SAFETY INFORMATION
RISK OF SERIOUS INFECTIONS:
Patients treated with TYENNE® (tocilizumab-aazg) are at increased risk for developing serious infections that may lead to hospitalization or death, including tuberculosis (TB), bacterial, invasive fungal, viral, or other opportunistic infections. If a serious infection develops, interrupt TYENNE until the infection is controlled.
Reported infections include:
- Active tuberculosis, which may present with pulmonary or extrapulmonary disease. Patients should be tested for latent tuberculosis before TYENNE use and during therapy. Treatment for latent infection should be initiated prior to TYENNE use.
- Invasive fungal infections, including candidiasis, aspergillosis, and pneumocystis. Patients with invasive fungal infections may present with disseminated, rather than localized, disease.
- Bacterial, viral and other infections due to opportunistic pathogens.
The risks and benefits of treatment with TYENNE should be carefully considered prior to initiating therapy in patients with chronic or recurrent infection.
Patients should be closely monitored for the development of signs and symptoms of infection during and after treatment with TYENNE, including the possible development of tuberculosis in patients who tested negative for latent tuberculosis infection prior to initiating therapy.
CONTRAINDICATION
TYENNE is contraindicated in patients with known hypersensitivity to tocilizumab products.
WARNINGS AND PRECAUTIONS
Gastrointestinal Perforations
Events of gastrointestinal (GI) perforation have been reported in clinical trials, primarily as complications of diverticulitis in patients treated with tocilizumab. Use TYENNE with caution in patients who may be at increased risk for GI perforation. Promptly evaluate patients presenting with new-onset abdominal symptoms for early identification of GI perforation.
Hepatotoxicity
Serious cases of hepatic injury have been observed in patients taking intravenous or subcutaneous tocilizumab products. Some of these cases have resulted in liver transplant or death. Time to onset for cases ranged from months to years after treatment initiation. Most cases presented with marked elevations of transaminases (> 5 times ULN), and some cases presented with signs or symptoms of liver dysfunction and only mildly elevated transaminases.
Treatment with tocilizumab was associated with a higher incidence of transaminase elevations; increased frequency and magnitude of these elevations were observed when tocilizumab was used in combination with potentially hepatotoxic drugs (e.g., methotrexate).
It is not recommended to initiate TYENNE treatment in RA, GCA, PJIA, and SJIA patients with elevated transaminases ALT or AST greater than 1.5x ULN. In patients who develop elevated ALT or AST greater than 5x ULN discontinue TYENNE.
Measure liver tests promptly in patients who report symptoms that may indicate liver injury. If the patient is found to have abnormal liver tests, TYENNE treatment should be interrupted. TYENNE should only be restarted in patients with another explanation for the liver test abnormalities after normalization of the liver tests.
Laboratory Parameters
Laboratory monitoring is recommended due to potential consequences of treatment-related laboratory abnormalities in neutrophils, platelets, lipids, and liver function tests. Dosage modifications may be required.
Neutropenia: Treatment with tocilizumab products was associated with a higher incidence of neutropenia. It is not recommended to initiate TYENNE treatment in RA, GCA, PJIA, and SJIA patients with a low neutrophil count i.e., absolute neutrophil count (ANC) less than 2000 per mm3. In patients who develop an ANC less than 500 per mm3 treatment is not recommended.
Thrombocytopenia: Treatment with tocilizumab products was associated with a reduction in platelet counts. It is not recommended to initiate TYENNE in RA, GCA, PJIA, and SJIA patients with a platelet count below 100,000 per mm3. In patients who develop a platelet count less than 50,000 per mm3, treatment is not recommended.
Elevated Liver Enzymes: It is not recommended to initiate TYENNE treatment in patients with elevated transaminases ALT or AST >1.5x ULN. In patients who develop elevated ALT or AST >5x ULN, treatment is not recommended.
Lipid Abnormalities: Treatment with tocilizumab products was associated with increases in lipid parameters such as total cholesterol, triglycerides, LDL cholesterols, and/or HDL cholesterol.
Immunosuppression
The impact of treatment with tocilizumab products on the development of malignancies is not known, but malignancies were observed in clinical studies with tocilizumab. TYENNE is an immunosuppressant, and treatment with immunosuppressants may result in an increased risk of malignancies.
Hypersensitivity Reactions
Hypersensitivity reactions, including anaphylaxis, have been reported in association with tocilizumab products and anaphylactic events with a fatal outcome have been reported with intravenous infusion of tocilizumab products. TYENNE for intravenous use should only be infused by a healthcare professional with appropriate medical support to manage anaphylaxis. For TYENNE subcutaneous injection, advise patients to seek immediate medical attention if they experience any symptoms of a hypersensitivity reaction. If anaphylaxis or other hypersensitivity reaction occurs, stop administration of TYENNE immediately and discontinue TYENNE permanently. Do not administer TYENNE to patients with known hypersensitivity to tocilizumab products.
Demyelinating Disorders
The impact of treatment with tocilizumab products on demyelinating disorders is not known, but multiple sclerosis and chronic inflammatory demyelinating polyneuropathy were reported rarely in clinical studies. Monitor patients for signs and symptoms of demyelinating disorders. Prescribers should exercise caution in considering the use of TYENNE in patients with preexisting or recent-onset demyelinating disorders.
Active Hepatic Disease and Hepatic Impairment
Treatment with TYENNE is not recommended in patients with active hepatic disease or hepatic impairment.
Vaccinations
Avoid use of live vaccines concurrently with TYENNE. No data are available on the secondary transmission of infection from persons receiving live vaccines to patients receiving TYENNE or on the effectiveness of vaccination in patients receiving TYENNE. Patients should be brought up to date on all recommended vaccinations prior to initiation of TYENNE therapy, if possible.
ADVERSE REACTIONS
Most common adverse reactions (incidence of at least 5%): upper respiratory tract infections, nasopharyngitis, headache, hypertension, increased ALT, injection site reactions.
DRUG REACTIONS
In GCA patients, no effect of concomitant corticosteroid on tocilizumab exposure was observed.
Cytochrome P450s in the liver are down-regulated by infection and inflammation stimuli including cytokines such as IL-6. Inhibition of IL-6 signaling in RA patients treated with tocilizumab products may restore CYP450 activities to higher levels than those in the absence of tocilizumab products leading to increased metabolism of drugs that are CYP450 substrates.
Exercise caution when coadministering TYENNE with CYP3A4 substrate drugs where decrease in effectiveness is undesirable, e.g., oral contraceptives, lovastatin, atorvastatin, etc.
USE IN PREGNANCY
The limited available data with tocilizumab products in pregnant women are not sufficient to determine whether there is a drug-associated risk for major birth defects and miscarriage.
You may report side effects to the FDA at (800) FDA-1088 or www.fda.gov/medwatch. You may also report side effects to Fresenius Kabi at (800) 551-7176.
Please see additional Important Safety Information in full Prescribing Information, including BOXED WARNING.